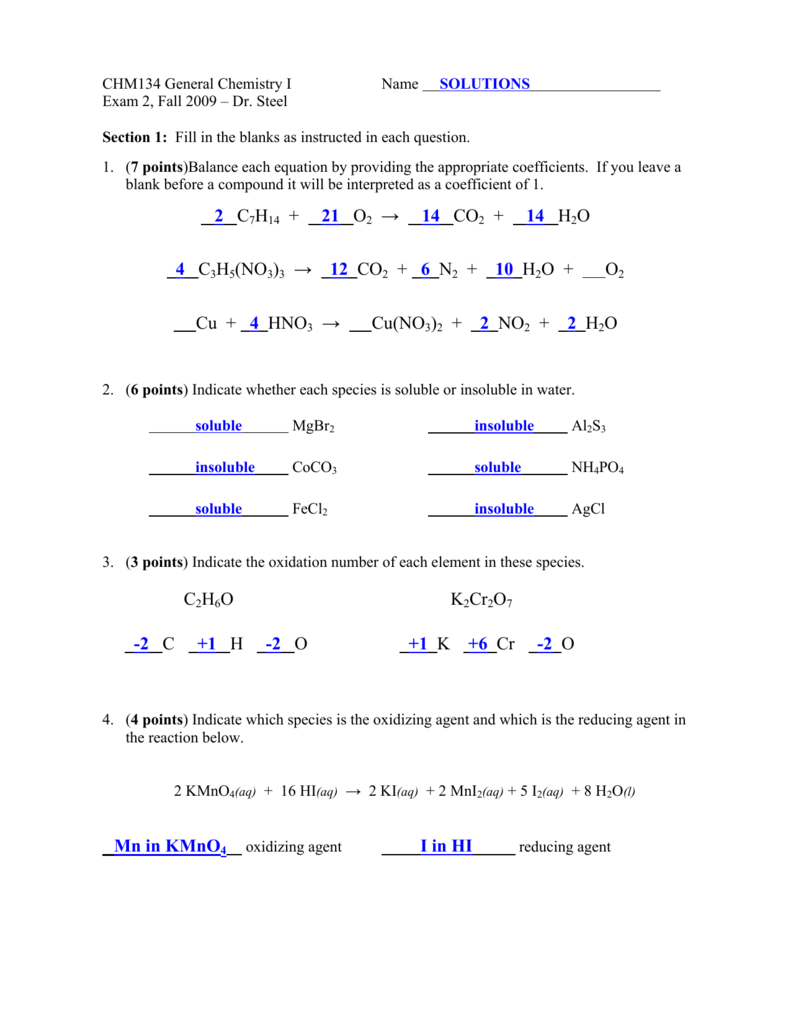
Which Element Is Oxidized in the Reaction Below
Answer Due to the iron hydrogen and chromium diluted. 1 Which element is oxidized in the reaction below.
Balancing Redox Reactions In Acidic And Basic Conditions Youtube
In the following chemical reaction which element is the oxidizing agent.

. Hence the oxidation state of Fe in product Fe CO₄I₂ s is 2. Fe CO5 0 2H1 9 Fe CO42 s CO 9 H2 9 2 Which element is oxidized in the reaction below. In the electrochemical cell using the redox reaction below the oxidation half reaction is _____.
Given the reaction. In the reaction shown below what species is oxidized. The element whose formal oxidation number decreases during a reaction is said to have been reduced.
In the following reaction which element in what species is oxidized. Which element is oxidized in the reaction below. 2 points a K2CrO4 BaCl2 BaCrO4 2KCI b Pb22 2Br PbBr c CuS Cus A a only Bb only C c only D a and c E b and c 3.
Tin is the element reduced. See the answer See the answer done loading. By assigning oxidation numbers we can pick out the oxidation and reduction halves of the reaction.
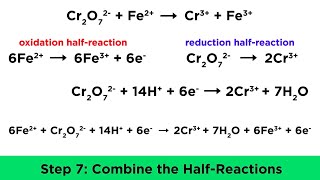
Fe2 h cr2o7-2 fe3 cr3 h2o. Xenon is the element oxidized. 2 points Fe2 H Cr2O72- Fe3 Cr3 H20 A Cr B O CH D Fe 2.
CH 4 2O2 CO2 2H 2O. For a simple combustion reaction. In this reaction Sn² gains two electrons to become Sn.
Which of the following reactions is a redox reaction. C Define which species are the reducing agents. 2H s Sn s Sn2 aq H2g Sn2eH2.
Chemistry use oxidation states to identify the element that is being oxidized and the element that is being reduced in the redox reaction Sn 4HNO3 --- SnO2 4NO2 2H2O I see that SN is. Fe CO5 l 2HI g -- Fe CO4I2 s CO g H2 g a. AuNO₃₃ aq 3 Lis Aus 3 LiNO₃ aq.
The oxidation number of reactants C I V H I and O 0 because oxygen. Tin is the element reduced. The element which is oxidized in the reaction below that is Fe Co5 l 2 Hi g Fe CO4 i2 s Co gH2 g is Fe this is because fe in Fe CO5 compound move from oxidation state of Positive five 5 to oxidation state of positive six 6 in Fe CO4i2 compound.
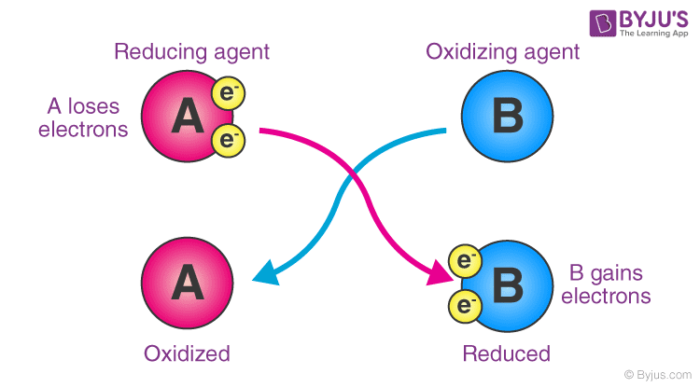
3 SnNO₃₂aq 2 Fes 2 FeNO₃₃aq 3 Sns Oxidizing agents gain electrons and are reduced. 34 Sn2 O3l2 0 Xe 2 O1 Hl 1 H6 Xe2 Ol 4 32 Sn2 O2l2. Oles of N2O gas are infused into a 100 L vessel at 300.
Which element is oxidized in the reaction below. Which statement best describes what taking place adminSend emailDecember 11 2021 minutes read You are watching consider the half reaction below. FeCO5 1 2HI g FeCO42 s CO g H2g H.
Which element is oxidized in the reaction below. Which element is oxidized and which is reduced in the reaction below. Which element is oxidized in the reaction below.
Redox Reactions Name _ Date Analyze the following redox reactions using the steps below. Aluminum is oxidized to Al 2 O 3 in this reaction which means that Fe 2 O 3 must be the oxidizing agent. X 0 - 2 0.
Civil Engineering questions and answers. Which element is oxidized and which is reduced in the reaction below. KCalculate the pressure inside the vessel at 100.
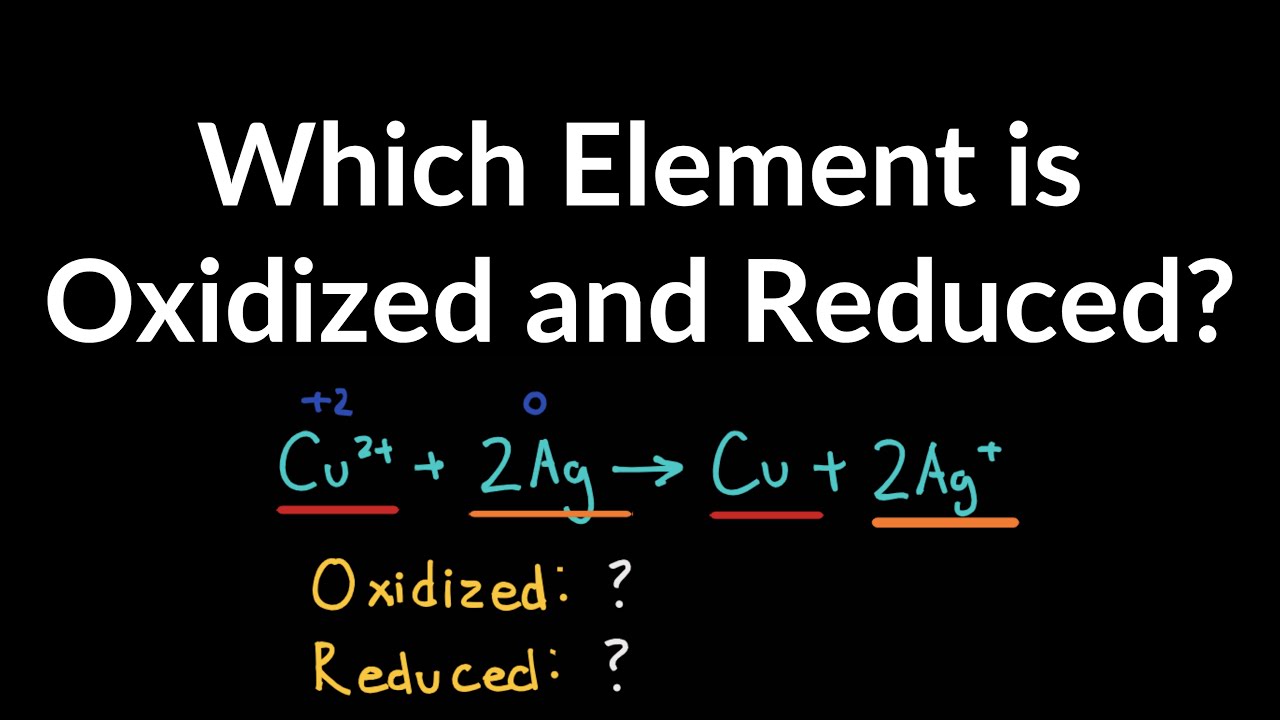
Which element is oxidized in the following reactionCr2O72- 6S2O32- 14H 2Cr3 3S4O62- 7H2O S Calculate the number of grams of aluminum produced in 100 hour by electrolysis of AlCl3 at a current of 100 A. Conversely Fe 2 O 3 is reduced to iron metal which means that aluminum must be the reducing agent. Solution for Which element is oxidized in the reaction below.
Y 50 0. Seconds after the start of the reaction. Same as we can find the oxidation state y of Fe in Fe CO₅ s.
Solved 1 Which element is oxidized in the reaction below. A Assign oxidation numbers to all atoms b Define which species have been oxidized and reduced. C3H8g 5O2g 3CO2g 4H2Og What is the total volume of H2Og formed when 800 liters of C3H8g is completely oxidized.
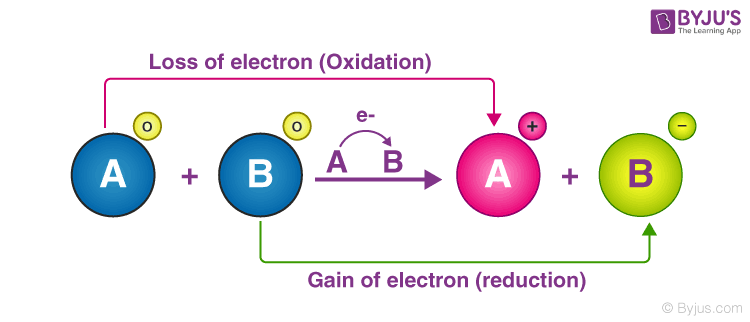
I- MnO4- H I2 MnO2 cO. The element whose formal oxidation number increases during a reaction is said to have been oxidized. 2NaI Br2 2NaBr I2.
- MnO4- 12 MnO2 H20 3 Which transformation. Temperature is held constant. Fe is oxidized by Hi while Hi itself is reduced by Fetherefore Fe act as a reducing agent.
The N2O decomposition 2 N2O g 2 N2 g O2 g is a zero order reaction that has a reaction rate constant of 200 103 Ms. Which statement best describes what taking place FAQconsider the half reaction below.

Alkanes Vs Alkenes Vs Alkynes Chemistry Lessons Chemistry Basics Organic Chemistry Study

Pin Ot Polzovatelya Elizabeth Na Doske Chemistry Obuchenie Himii Organicheskaya Himiya Matematicheskie Bloknoty
Oxidizing Agent Definition Properties Examples Applications

Electricity In 2020 Science Notes Electricity Elementary Science

Chemistry Redox Reactions Physics And Mathematics

10 Perfect Types Of Jump Rings For Jewelry Making Craft Minute Jewelry Making Bracelet Jewelry Making Bracelet Making

Using Oxidation Numbers To Identify Oxidation And Reduction Worked Example Video Khan Academy

Dynamics Of Low Temperature N2o Formation Under Scr Reaction Conditions Over A Cu Ssz 13 Catalyst Sciencedirect

Solved Name 1 Which Element Is Oxidized In The Reaction Chegg Com

Electricity Electricity Science Notes Elementary Science

How To Find The Oxidizing And Reducing Agent Youtube

Electricity Electricity Science Notes Elementary Science

Solved Name 1 Which Element Is Oxidized In The Reaction Chegg Com
What Is A Redox Reaction Explain With An Example

Oxidation Reduction Reaction Oxidation States Britannica
How To Identify Oxidized And Reduced Element In Redox Reaction With Examples And Problems Youtube

Chemistry Reduction And Oxidation Reactions Wikiversity

Pin By Tanji On Chemistry Education Chemistry Lessons Chemistry Classroom Chemistry Basics
Comments
Post a Comment